how many valence electrons does ag have|3.1: Valence Electrons : Tagatay Mar 23, 2023
Here's the ITV Racing Schedule as we head to Haydock on Saturday, September 7 (see our TV Guide for full listings). Ed Chamberlin and Francesca Cumani present coverage of a meeting headlined by the Sprint Cup at Haydock Park. Plus, racing from Kempton and Ascot. Are you traveling outside the UK? You .
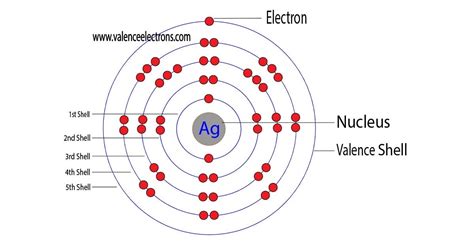
how many valence electrons does ag have,The ground-state electron configuration of silver is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. This electron configuration shows that the last shell of silver has an electron and the d-orbital has a total of ten electrons. Therefore, the valence electronsof silver are one. The elements that form bonds by donating . Tingnan ang higit paThe total number of electrons in silver is forty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in silver in . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa For Ag this means we have 11 valence electrons. However since Silver only forms 1+ ions it could be argued that the 5s1 represent the valance electron for Ag. Note that for transition.
Mar 23, 2023

The web page lists the valences of the elements from hydrogen to .Learn how to determine the number of valence electrons for an element using the periodic table. Ag (silver) has one valence electron in group 1. See patterns, diagrams, and .
Learn how valence electrons determine chemical properties and bonding of elements. Find out how many valence electrons each element has by looking at its Group in the periodic table. How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so .
Learn how to identify valence electrons, the electrons in the outermost shell of an atom, and see examples of oxygen, copper, and fluorine. Watch the video and read the comments .Four covalent bonds. Carbon has four valence electrons and here a valence of four. Each hydrogen atom has one valence electron and is univalent. In chemistry and physics, .
Silver is the 47th element of the periodic table so its atomic number is 47. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a silver atom has forty-seven .3.1: Valence Electrons sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 3d 10 4p 1, which contains three valence electrons (underlined). The completely filled d orbitals count as core, not valence, electrons. Transition elements or transition metals. That is why the electron configuration for (Ag) silver should end as 4 d 9, and hence it is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 9. How Many Valence Electrons Does Silver Have. Silver has only one valence electron in its outer shell. See the picture below for more information. Silver Number of Valence Electrons Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd . Ag: [Kr]4d105s1. The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of the periodic table or the 9th column of the transition metal or d block. Therefore th electron configuration for silver must end as 4d^9, 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 .
The valence (or valency) of an element is a measure of its combining power with other atoms when it forms chemical compounds or molecules. The concept of valence was developed in the last half of the 19th century and was successful in explaining the molecular structure of many organic compounds. The quest for the underlying causes of valence .Answer and Explanation: 1. Become a Study.com member to unlock this answer! Create your account. View this answer. Silver has one valence electron, a 5 s electron. Electrons in the outermost energy level of an atom are known as valence electrons as they are in the. See full answer below. 1s^2 doesn't mean squared here, it means that there are 2 electrons in the 1s orbital. I don't know what level of education you're at so I don't want to confuse you too much, but we know .

SILVER. No, silver does not have any more than #1# valence electron.. We only count valence electrons as those electrons that are important in chemical bonding (here, the one #5s# electron): #[Kr] 4d^10 5s^1# We never see silver with an oxidation state higher than #+1# (which would in principle require transferring one valence . How Many Valence Electrons Does Ag Have. 23 How. February 5, 2023 January 1, 2023 Tony. The number of valence electrons in an atom is determined by the number of protons in its nucleus. The element silver (Ag) has 47 protons, so it has 47 valence electrons.How many valence electrons does magnesium ion(Mg +2) have? After the electron configuration, the last shell of the magnesium atom has two electrons. In this case, the valency of magnesium is 2. We know the details about this. The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation.How many valence electrons does a Silver atom have? Silver has 1 valence electrons. Silver has 47 electrons out of which 1 valence electrons are present in the 4d10 5s1 outer orbitals of atom. What is the melting Point of Silver? Melting Point of Silver is 1234.93 K. What is the boiling Point of Silver? Boiling Point of Silver is 2435 K.
how many valence electrons does ag haveMany elements have a common valence related to their position in the . Chlorine has seven valence electrons and can form only one bond with an atom that donates a valence electron to complete . Ag: 48 Cd: 49 In: 50 Sn: 51 Sb: 52 Te: 53 I: 54 Xe: 6: 55 Cs: 56 Ba: 71 Lu: 72 Hf: 73 Ta: 74 W: 75 Re: 76 Os: 77 Ir: 78 Pt: 79 Au: 80 Hg: 81 Tl: 82 .how many valence electrons does ag have 3.1: Valence Electrons Many elements have a common valence related to their position in the . Chlorine has seven valence electrons and can form only one bond with an atom that donates a valence electron to complete . Ag: 48 Cd: 49 In: 50 Sn: 51 Sb: 52 Te: 53 I: 54 Xe: 6: 55 Cs: 56 Ba: 71 Lu: 72 Hf: 73 Ta: 74 W: 75 Re: 76 Os: 77 Ir: 78 Pt: 79 Au: 80 Hg: 81 Tl: 82 . When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most .Step-1: Determining the total number of electrons in argon. 1st we need to know the total number of electrons in the argon atom. To know the number of electrons, you need to know the number of protons in argon. And to know the number of protons, you need to know the atomic number of the argon element.
how many valence electrons does ag have|3.1: Valence Electrons
PH0 · Valences of the Elements Chemistry Table
PH1 · Valence electrons (video)
PH2 · Valence electron
PH3 · Valence Electrons Chart for All Elements
PH4 · Valence Electrons
PH5 · How to Find the Valence Electrons for Silver (Ag)?
PH6 · How to Find the Valence Electrons for Silver (Ag)
PH7 · Electron Configuration for Silver (Ag and Ag+ ion)
PH8 · Electron Configuration for Silver (Ag and Ag+ ion)
PH9 · Determine valence electrons using the periodic table
PH10 · 3.1: Valence Electrons